12+ Stoichiometry Chapter 9
It is not an. Solution The data from Figure 122 are tabulated below and a plot of lnH 2 O 2 is shown in Figure 129.
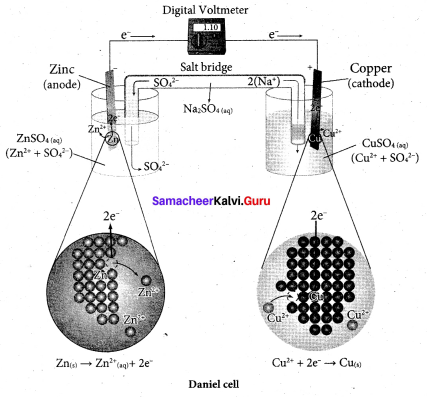
Search Results For 9 1 2 Samacheer Kalvi
Web As an example of the processes depicted in this figure consider a sample of water.

. And porosities are crucial for the results of corrosion tests. Types of Chemical Reactions. Limiting Reagent and Percent Yield.
How would the graph in Figure 912 change if the number of moles of gas in the sample used to determine the curve were doubled. Web United Nations Sustainable Development Goals - Time for Global Action for People and Planet. 93 Stoichiometry of Gaseous Substances Mixtures and Reactions.
Web Stoichiometry is a way to use mole ratios and molar masses to determine the amount of a substance when given the amount of another substance. Web Chapter 9Chemical Names and Formulas. Macromolecules with fewer than 50 amino acids are known as peptides.
The word stoichiometry is derived from the Greek word stoikhein meaning element and metron meaning measure. Web When a mixture of ethane and oxygen in the ratio 91 by volume is compressed to about 120 atm pressure and passed over copper tubes at 475K ethyl alcohol is formed. 15149 Energy balance constant pressure The energy balance for the constant-pressure case follows from Equation 615 C P dT.
Web 95 Stoichiometry of Reactions Involving Gases. Web 12 12 3 9 21 19 19. Class 12 NCERT Solutions Chemistry Chapter 4 - Chemical Kinetics is fundamental to many of the concepts that you will study in the future.
These concepts are combined with the work-energy theorem to provide a convenient means of analyzing an object or system of objects moving between an initial and final state. Web 4 Stoichiometry of Chemical Reactions. Given below are a few related subtopics that students should be aware of while studying Chapter 4 of Class 12 Chemistry.
Amorphous structures and non-ceramic chemicals frequently used as sintering aids are starting points of corrosive attack. 121 The Dissolution Process. The gas condenses forming liquid H 2 O.
For example liquid water forms on the outside of a cold. Web Concepts of work kinetic energy and potential energy are discussed. Web Subtopics for Class 12 Chemistry Chapter 4 Chemical Kinetics.
Web Proteins are very large molecules containing many amino acid residues linked together in very specific order. French nobleman Antoine Lavoisier widely regarded as the father of modern chemistry changed chemistry from a qualitative to a quantitative science through his work with gasesHe discovered the law of conservation. Web Join an activity with your class and find or create your own quizzes and flashcards.
BYJUS is Indias largest ed-tech company and the creator of Indias most loved school learning app. Less impurities and exact stoichiometry lead to less corrosion. Launched in 2015 BYJUS offers highly personalised and effective learning programs for classes 1 - 12 K-12 and aspirants of competitive exams like.
The Arithmetic of Equations. Web Show that the data in Figure 122 can be represented by a first-order rate law by graphing lnH 2 O 2 versus time. 41 Writing and Balancing Chemical Equations.
Web The Code of Federal Regulations CFR is the official legal print publication containing the codification of the general and permanent rules published in the Federal Register by the departments and agencies of the Federal Government. Dimers also have significant implications in polymer chemistry inorganic chemistry and biochemistry. 42 Classifying Chemical Reactions.
Figure 114 Peptides and Proteins are macromolecules built from. Web Chemical Stoichiometry refers to the quantitative study of the reactants and products involved in a chemical reaction. Web Class 11 is a crucial and significant year for high school students because it is during this year that you set the pedestal for all the essential topics and concepts you will cover in the Class 12 board exams.
Determine the rate constant for the decomposition of H 2 O 2 from these data. IPad Android and Kindle version. These problems always have to start with a balanced.
When gaseous water is cooled sufficiently the attractions between H 2 O molecules will be capable of holding them together when they come into contact with each other. 96 Effusion and Diffusion of Gases. Naming and Writing Formulas for Ionic Compounds.
French nobleman Antoine Lavoisier widely regarded as the father of modern chemistry changed chemistry from a qualitative to a quantitative science through his work with gasesHe discovered the law of conservation. 97 The Kinetic-Molecular Theory. Rate of a Chemical.
Checklist for Chapter 9. Student Study Guide Chapter 9. Written by teachers for teachers and students The Physics Classroom provides a wealth of resources that meets the varied needs of both.
2B stoichiometry we substitute the rate expression and 1 into Equation 617 to obtain C V dT dt H R RT kn A in which C V V R CV is the total constant-volume heat capacity. Proteins range in size from 50 amino acids in length to the largest known protein containing 33423 amino acids. 98 Non-Ideal Gas Behavior.
Figure 923 Sideways overlap of p. Web The study of the chemical behavior of gases was part of the basis of perhaps the most fundamental chemical revolution in history. In large pumps with shafts 100350 mm.
Web Figure 922 Head-to-head overlap of p orbitals Sideways overlap of the remaining four p atomic orbitals can occur along the two other axes generating four π molecular orbitals having electron density on opposite sides of the internuclear axis Figure 923 Sideways overlap of p orbitals. Web A dimer ˈ d aɪ m ər di- two -mer parts is an oligomer consisting of two monomers joined by bonds that can be either strong or weak covalent or intermolecular. Web The Physics Classroom serves students teachers and classrooms by providing classroom-ready resources that utilize an easy-to-understand language that makes learning interactive and multi-dimensional.
2C 2 H 6 O 2 2C 2 H 5 OH When a mixture of ethane and oxygen is passed through heated MoO the mixture is oxidized to ethanal. For the A. Web The study of the chemical behavior of gases was part of the basis of perhaps the most fundamental chemical revolution in history.
The term Stoichiometry was first coined or discovered by a German chemist named Jeremias Richter. A brief comprehension of the chapter - some basic concepts of chemistry can help you understand and appreciate the role of chemistry in different. The Electronic Code of Federal Regulations eCFR is a continuously updated online version of the CFR.
An Introduction to Oxidation-Reduction Reactions. The term homodimer is used when the two molecules are identical eg.

Some Basic Concept Of Chemistry Worksheet

Characterizing The Structure And Oligomerization Of Major Royal Jelly Protein 1 Mrjp1 By Mass Spectrometry And Complementary Biophysical Tools Abstract Europe Pmc
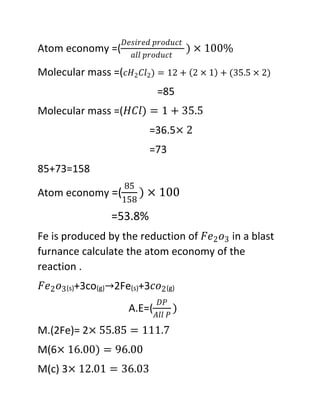
Atom Economy
Chapter 9 Kyla S Chemistry
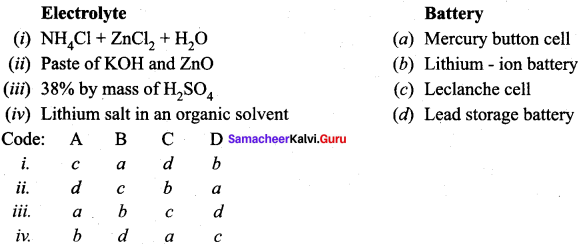
Search Results For 9 1 2 Samacheer Kalvi

4 3 Limiting Reactant Theoretical Yield And Percent Yield Chemistry Libretexts

Modern Chemistry Chapter 9 Stoichiometry Ppt Download
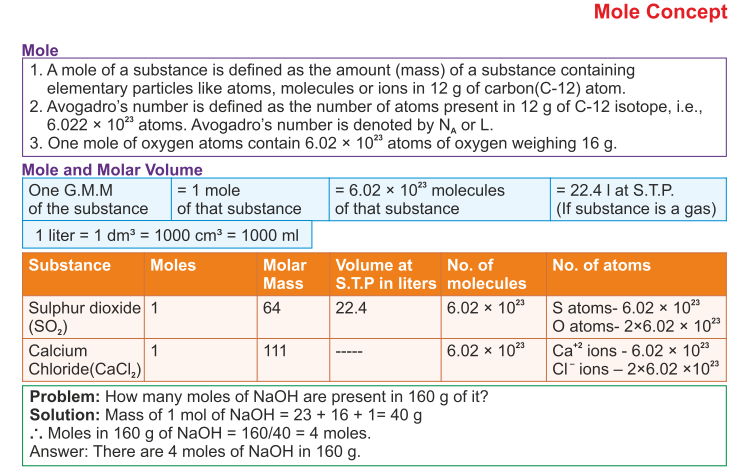
Icse Solutions For Class 10 Chemistry Mole Concept And Stoichiometry A Plus Topper

Modern Chemistry Chapter 9 Stoichiometry Ppt Download

Pdf Stoichiometry Notes Wooyoung Jeong Academia Edu
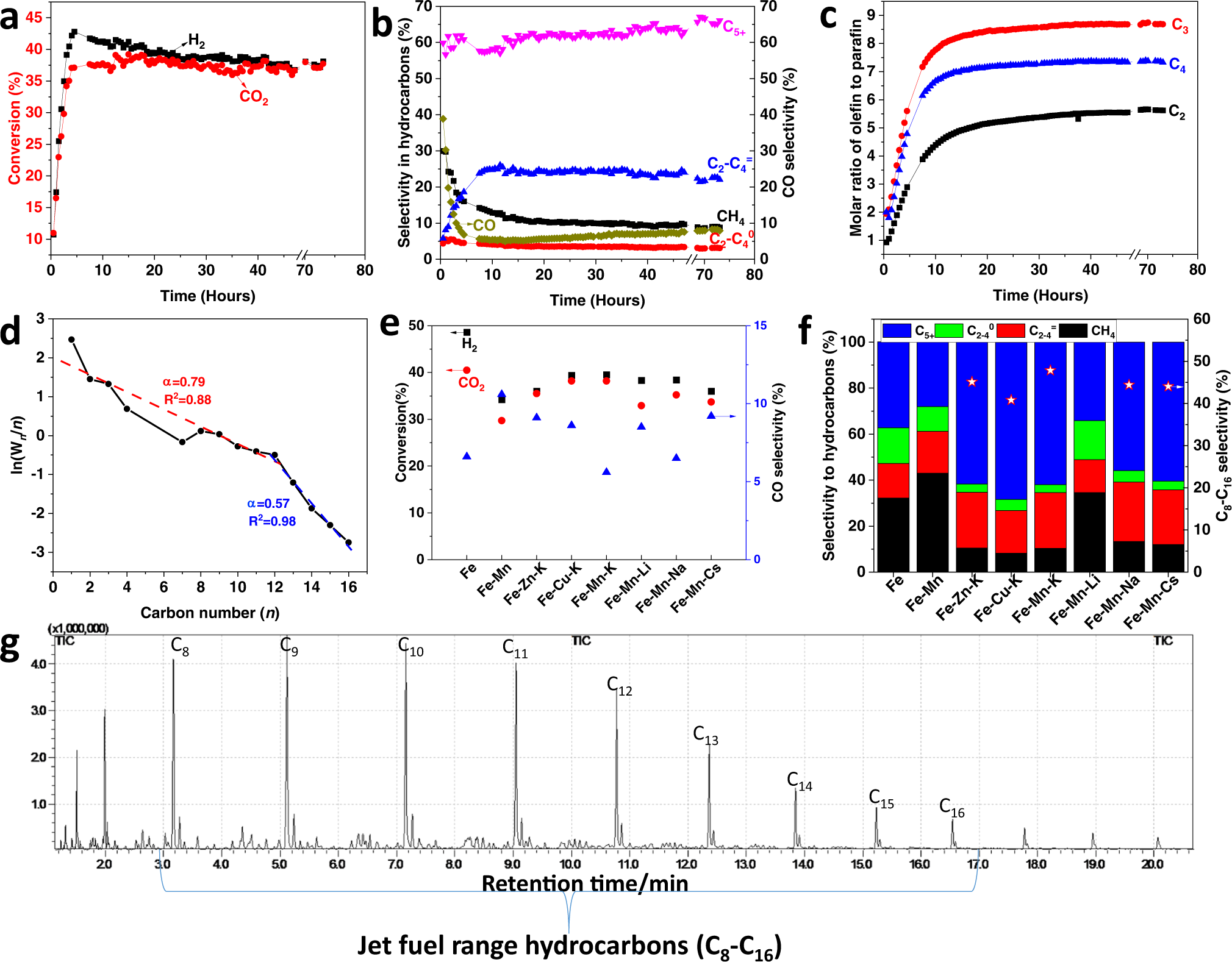
Transforming Carbon Dioxide Into Jet Fuel Using An Organic Combustion Synthesized Fe Mn K Catalyst Nature Communications
Using Ch4 2o2 Co2 2h2o How Many Grams Of Co2 Will Be Produced Quora

Solved Need Help With Problem 6 Through 13 Can The Problems Chegg Com

Chemistry The Science In Context Volume I And Ii 4th Edition Gilbert

Modern Chemistry Chapter 9 Stoichiometry Ppt Download

Chapter 9 Stoichiometry Pages Intro To Stoichiometry All Stoichiometric Calculations Start With A To Solve You Ppt Download
Chapter 11 Stoichiometry 11 1 Defining Stoichiometry